Press release
First Patient Enrolled in Abiomed’s RECOVER IV Randomized Controlled Trial of Impella® as a Therapy for AMI Cardiogenic Shock
First Trial in AMI Cardiogenic Shock to Use the FDA’s EFIC Protocol
DANVERS, Mass. – Nov. 27, 2023 – Abiomed, part of Johnson & Johnson MedTech1, announces the first patient in the world has been enrolled in the landmark RECOVER IV randomized controlled trial (RCT). The on-label, two-arm trial will randomize 548 patients to assess whether Impella support prior to percutaneous coronary intervention (PCI) is superior to PCI without Impella in patients with acute myocardial infarction (AMI) cardiogenic shock.
Impella is the only mechanical circulatory support device for the treatment of AMI cardiogenic shock with premarket approval from the United States Food and Drug Administration. Multiples studies, including the Inova Study2, the National Cardiogenic Shock Initiative (NCSI) Study3 and the Japanese J-PVAD Study4, have demonstrated significant improvement in AMI cardiogenic shock survival rates when Impella and best practices are implemented, including the use of Impella prior to PCI and identifying shock early. (See figure 1).
“Fifteen years of clinical studies have helped us establish best practices and shown that Impella increases survival and heart recovery in AMI cardiogenic shock patients,” said Chuck Simonton, MD, Abiomed’s chief medical officer. “The design of RECOVER IV, which incorporates those best practices, is intended to further validate the benefits of Impella utilization in AMI cardiogenic shock patients and provide evidence to achieve a Class 1 guideline recommendation.”
This first patient was enrolled at New Mexico Heart Institute by Mark Bieniarz, MD5, an interventional cardiologist and the principal investigator for RECOVER IV at New Mexico Heart Institute. RECOVER IV is the first trial within the field of AMI cardiogenic shock to use the U.S. Food and Drug Administration’s (FDA) exception from informed consent (EFIC) protocol, an emergency research regulation. Since AMI cardiogenic shock patients are often too sick to provide consent, it has been difficult to study this patient population . Emergency research regulations require study investigators to consult and disclose information about the study with representatives from the community where the research will take place. EFIC allows for patients to be enrolled prior to obtaining traditional consent.
“We are thrilled to be the first site to enroll a patient into the historic RECOVER IV trial,” said Dr. Bieniarz. “The EFIC process provides us a pathway to successfully enroll patients and compare outcomes in patients who receive Impella support for acute myocardial infarction cardiogenic shock and those patients who do not receive Impella support for the same condition.”
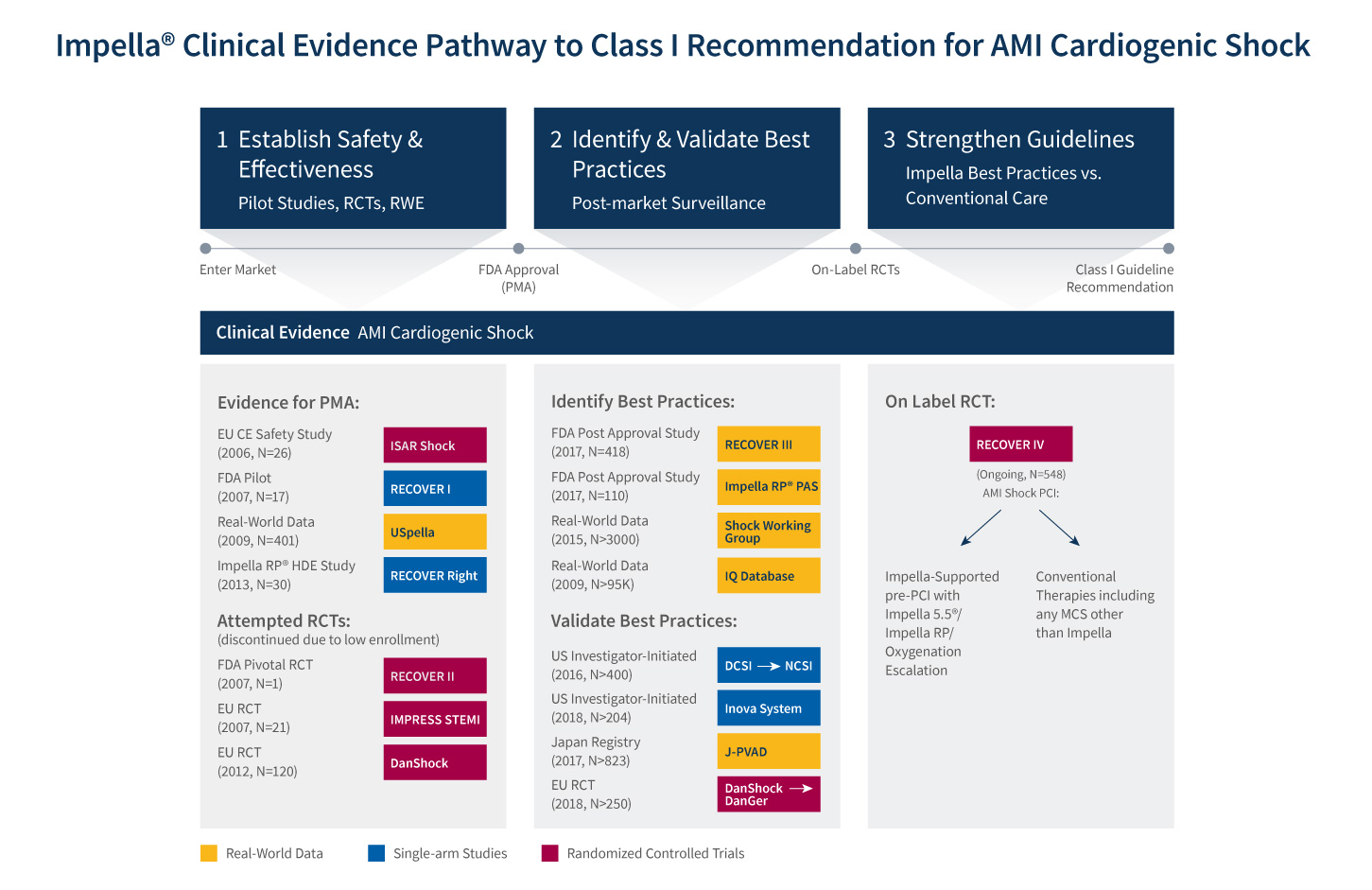
The primary endpoint of RECOVER IV is all-cause mortality at 30 days. Secondary endpoints include major adverse cardiovascular and cerebrovascular events at 30 days, days alive out of the hospital at six months, recovery of left ventricular (LV) function, need for durable ventricular assist device or heart transplant, and health-related quality of life as measured by responses to the Kansas City Cardiomyopathy Questionnaire at one year. Abiomed’s goal in conducting the RECOVER IV RCT is to achieve AMI cardiogenic shock Class I guideline recommendations for Impella use in AMI cardiogenic shock. (See figure 2).
For more information about RECOVER IV, click here.
About Abiomed
Based in Danvers, Massachusetts, USA, Abiomed, part of Johnson & Johnson MedTech, is a leading provider of medical technology that provides circulatory support and oxygenation. Our products are designed to enable the heart to rest and recover by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit www.abiomed.com.
About Johnson & Johnson MedTech
At Johnson & Johnson MedTech, we unleash diverse healthcare expertise, purposeful technology, and a passion for people to transform the future of medical intervention and empower everyone to live their best life possible. For more than a century, we have driven breakthrough scientific innovation to address unmet needs and reimagine health. In surgery, orthopaedics, vision, and interventional solutions, we continue to help save lives and create a future where healthcare solutions are smarter, less invasive, and more personalized.
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding Abiomed technology. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Abiomed, Inc. and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns of purchasers of health care products and services; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended January 1, 2023, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. None of Abiomed, Inc. nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
For further information please contact:
Jenny Leary
Associate Director, US Communications
[email protected]
978-882-8491
Figure 1: Impella Improves Survival and Heart Recovery
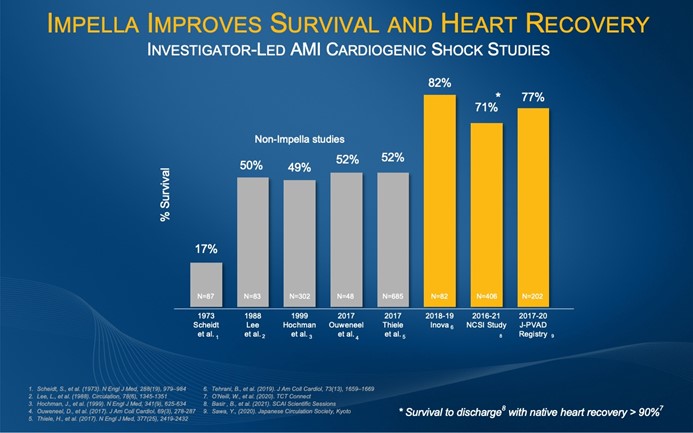
Figure 2: Impella Clinical Evidence Pathway to Class I Recommendation for AMI Cardiogenic Shock
References
- Abiomed, Inc. is part of Johnson & Johnson MedTech, which comprises the surgery, orthopedics, vision and interventional solutions businesses within Johnson & Johnson's MedTech segment.
- Tehrani, B., et al. (2019). J Am Coll Cardiol, 73(13), 1659-1669
- Basir, B., et al. (2021). SCAI Scientific Sessions
- Sawa, Y., (2020). Japanese Circulation Society, Kyoto
- Dr. Mark Bieniarz serves as PI for the RECOVER IV RCT at New Mexico Heart Institute. He is not compensated for his involvement with this research study or press release.