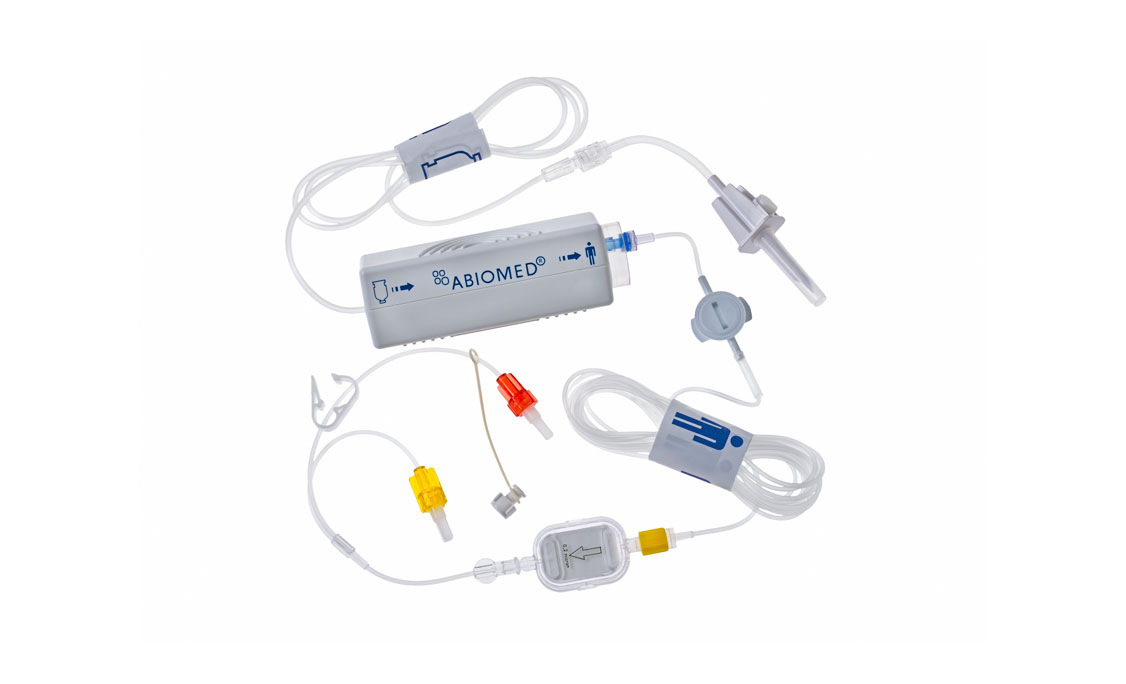
Impella RP®
Overview
Impella RP with SmartAssist is the only right heart pump equipped with dual-sensor technology to assist with pump management.
This technology pumps blood from the inferior vena cava to the pulmonary artery. The pump is inserted with venous access and advanced over a wire into the pulmonary artery using standard catheterization techniques.
- Provides circulatory assistance for up to 14 days in certain patients with a body surface area ≥ 1.5 m2
- Delivers flow of greater than 4.0 L/min of blood
- Enables biventricular support when the left side is already supported with a left-sided Impella®
Clinical Evidence
The prospective, multi-center FDA PMA post-approval study compared survival in patients who would have met enrollment criteria for the RECOVER RIGHT trial to those who would not have qualified for the trial because they were in cardiogenic shock for more than 48 hours. These results, from the Impella RP’s post-approval study, are similar to the survival rate in Impella RP’s pre-approval study, RECOVER RIGHT.2
- 73%
Survival rate for patients treated with Impella RP in the RECOVER RIGHT Studies*3
- 70%
Survival rate for patients treated with Impella RP in the PAS2
Navin Kapur, MD, shares an Impella RP case in which LV and aortic pressures increased after just a few heartbeats on support
Accessories
Additional Resources
Safety Information
The Impella RP® with SmartAssist® System is indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open‐heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.
The Impella RP with SmartAssist System is contraindicated for patients with the following conditions: Disorders of the pulmonary artery wall that would preclude placement or correct positioning of the Impella RP devices. Mechanical valves, severe valvular stenosis or valvular regurgitation of the tricuspid or pulmonary valve. Mural thrombus of the right atrium or vena cava. Anatomic conditions precluding insertion of the pump. Presence of a vena cava filter or caval interruption device, unless there is clear access from the femoral vein to the right atrium that is large enough to accommodate a 22Fr catheter.
The potential adverse effects (eg, complications) associated with the use of Impella RP with SmartAssist: Arrhythmia, Atrial fibrillation, Bleeding, Cardiac tamponade, Cardiogenic shock, Death, Device malfunction, Hemolysis, Hepatic failure, Insertion site infection, Perforation, Phlegmasia cerulea dolens (a severe form of deep venous thrombosis), Pulmonary valve insufficiency, Respiratory dysfunction, Sepsis, Thrombocytopenia, Thrombotic vascular (non-central nervous system) complication, Tricuspid valve injury, Vascular injury, Venous thrombosis, Ventricular fibrillation and/or tachycardia.
Footnotes
- Lala, A., et al., (2018). J Card Fail, 24(3), 148-156.
- Impella RP System PMA Post-Approval Study Final Report.
- Anderson, M., et al., (2018). The Journal of Heart and Lung Transplantation, 37(12), 1448-1458.
*RECOVER RIGHT + CAP + HDE PAS
Survival to 30 days after device explant or hospital discharge (whichever was longer), or to the start of next longer-term therapy.
IMP-1704